Findings accepted for publication within the official journal of the American School of Allergy, Bronchial asthma and Immunology
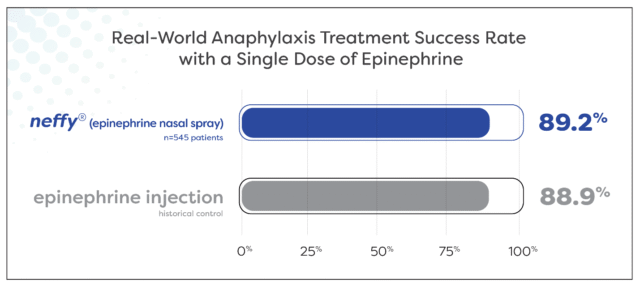
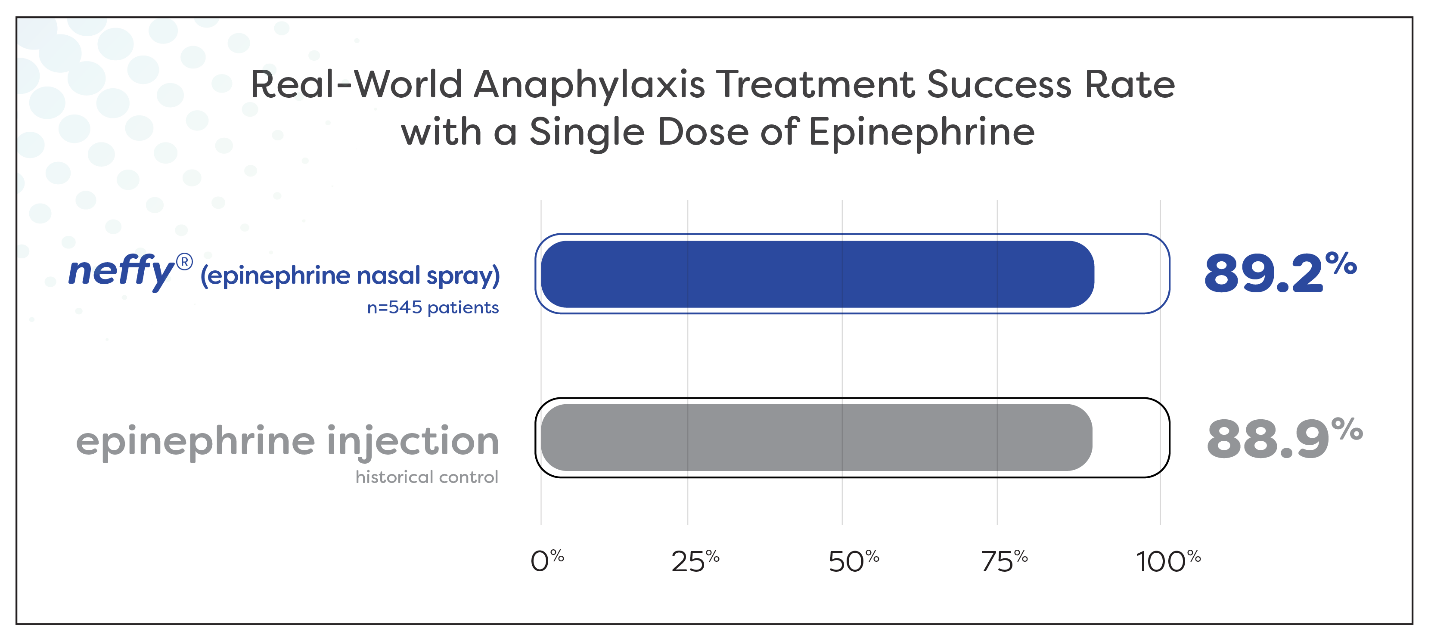
About 9 out of each 10 sufferers experiencing anaphylaxis have been successfully handled with a single dose of neffy
Outcomes counsel real-world effectiveness of neffy is in line with that traditionally reported for epinephrine injection
SAN DIEGO, Sept. 08, 2025 (GLOBE NEWSWIRE) — ARS Prescription drugs, Inc. (Nasdaq: SPRY), a business stage biopharmaceutical firm devoted to empowering at-risk sufferers and their caregivers to raised defend sufferers from allergic reactions that might result in anaphylaxis, at this time shares real-world proof evaluating the medical efficiency of neffy® (epinephrine nasal spray) in sufferers experiencing anaphylaxis signs throughout oral meals problem and allergen immunotherapy. These findings symbolize the primary large-scale evaluation of remedy outcomes with neffy throughout routine medical apply and was accepted in August for publishing as a correspondence within the Annals of Allergy, Bronchial asthma and Immunology, the official journal of the American School of Allergy, Bronchial asthma and Immunology.
Almost 90% (89.2%) of 545 sufferers experiencing anaphylaxis signs throughout oral meals problem and allergen immunotherapy have been efficiently handled with a single dose of neffy by a healthcare supplier. Meta-analyses report an analogous proportion of sufferers, 88.9%, being efficiently handled with a single dose of epinephrine intramuscular injection or auto-injector by a healthcare supplier for food-induced anaphylaxis.1 This extremely related remedy success charge helps that the real-world medical effectiveness of neffy in anaphylaxis is in line with epinephrine injection.
Importantly, these real-world knowledge construct upon beforehand revealed medical proof, together with a potential Section 3 examine2 (n = 15 sufferers) assessing the efficacy of neffy for the remedy of oral meals challenge-induced anaphylaxis signs. In that examine, no sufferers required a second dose of neffy for remedy of the preliminary anaphylactic response.
“These knowledge reinforce present findings and is the primary large-scale report of real-world remedy outcomes with neffy throughout anaphylaxis occasions. The discovering that about 9 out of each 10 sufferers have been efficiently handled with a single dose of neffy in additional than 500 sufferers is actually an identical to the historic response charges noticed with epinephrine injection,” mentioned Dr. Thomas B. Casale, M.D., Professor of Drugs and Pediatrics and Chief of Scientific and Translational Analysis within the USF Well being Morsani School of Drugs’s Division of Allergy and Immunology on the College of South Florida in Tampa, Florida. “We consider these real-world outcomes knowledge assist the medical interchangeability of neffy and epinephrine injection, constructing on the medical research carried out for FDA approval that confirmed neffy achieved blood ranges and pharmacodynamic responses inside the vary of accepted injection merchandise.”
Concerning the neffy Expertise Program
The info from this retrospective observational evaluation was collected from healthcare suppliers collaborating within the neffy expertise program. Within the neffy expertise program, healthcare suppliers got six doses of neffy to be used to rescue sufferers experiencing anaphylaxis signs throughout oral meals problem or allergen immunotherapy. neffy labeling states {that a} second dose ought to be administered if anaphylaxis signs proceed or worsen beginning 5 minutes after the primary dose.
As of the March 2025 knowledge cut-off, 301 healthcare suppliers had responded to the survey instrument, and a complete of 545 sufferers have been reported having been handled with neffy 2 mg. 4 hundred and eighty-six (486) of those sufferers had been efficiently handled with a single dose of neffy 2 mg, with the remaining 59 sufferers requiring a second dose of epinephrine. The neffy expertise program is actively ongoing and now consists of each the two mg and 1 mg doses.
This knowledge from the neffy expertise program is in line with medical trials just lately revealed on the real-world medical effectiveness of neffy. Nevertheless, this observational evaluation differs from a randomized medical trial in a number of methods. The research have completely different endpoints and there are inherent limitations in real-world observational research, together with lack of randomization, lack of uniform timing or sort of medical assessments and challenges with lacking knowledge.
About neffy®
neffy is a nasal spray used for emergency remedy of allergic reactions together with anaphylaxis, in adults and kids aged 4 years and older who weigh 33 lbs. or larger.
INDICATION AND IMPORTANT SAFETY INFORMATION FOR neffy (epinephrine nasal spray)
INDICATION
neffy is indicated for emergency remedy of sort I allergic reactions, together with anaphylaxis, in grownup and pediatric sufferers aged 4 years and older who weigh 33 lbs. or larger.
IMPORTANT SAFETY INFORMATION
neffy accommodates epinephrine, a medication used to deal with allergic emergencies (anaphylaxis). Anaphylaxis could be life-threatening, can occur in minutes, and could be attributable to stinging and biting bugs, allergy injections, meals, medicines, train, or different unknown causes.
At all times carry two neffy nasal sprays with you as a result of chances are you’ll not know when anaphylaxis might occur and since chances are you’ll want a second dose of neffy if signs proceed or come again. Every neffy accommodates a single dose of epinephrine. neffy is to be used within the nostril solely.
Use neffy straight away, as quickly as you discover signs of an allergic response. If signs proceed or worsen after the primary dose of neffy, a second dose is required. If wanted, administer a second dose utilizing a brand new neffy in the identical nostril beginning 5 minutes after the primary dose. Get emergency medical assist for additional remedy of the allergic emergency (anaphylaxis), if wanted after utilizing neffy.
Inform your healthcare supplier when you’ve got underlying structural or anatomical nasal situations, about all of the medicines you are taking, and about all of your medical situations, particularly when you’ve got coronary heart issues, kidney issues, low potassium in your blood, Parkinson’s illness, thyroid issues, hypertension, diabetes, are pregnant or plan to develop into pregnant, or plan to breastfeed.
Inform your healthcare supplier if you happen to take or use different nasal sprays or water tablets (diuretics) or if you happen to take medicines to deal with melancholy, irregular coronary heart beats, Parkinson’s illness, coronary heart illness, thyroid illness, medicines utilized in labor, and medicines to deal with allergy symptoms. neffy and different drugs might have an effect on one another, inflicting unwanted effects. neffy might have an effect on the way in which different medicines work, and different medicines might have an effect on how neffy works.
neffy might trigger critical unwanted effects. When you’ve got sure medical situations or take sure medicines, your situation might worsen, or you might have extra or longer lasting unwanted effects while you use neffy.
Widespread unwanted effects of neffy embrace: nasal discomfort, headache, throat irritation, chest and nasal congestion, feeling overly excited, nervous or anxious, nostril bleed, nostril ache, sneezing, runny nostril, dry nostril or throat, tingling sensation, together with within the nostril, feeling drained, dizziness, nausea, and vomiting.
Inform your healthcare supplier when you’ve got any unwanted effects that hassle you or that don’t go away after utilizing neffy.
These aren’t all the potential unwanted effects of neffy. Name your healthcare supplier for medical recommendation about unwanted effects. To report unwanted effects, contact ARS Prescription drugs Operations, Inc. at 1-877-MY-NEFFY (877-696-3339) or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see the complete Prescribing Data and Affected person Data for neffy.
About Sort I Allergic Reactions Together with Anaphylaxis
Sort I allergic reactions are critical and doubtlessly life-threatening occasions that may happen inside minutes of publicity to an allergen and require fast remedy with epinephrine, the one FDA-approved remedy for these reactions. Whereas epinephrine auto-injectors have been proven to be extremely efficient, there are effectively revealed limitations that end in many sufferers and caregivers delaying or not administering remedy in an emergency state of affairs. These limitations embrace concern of the needle, lack of portability, needle-related security issues, lack of reliability, and complexity of the units. There are roughly 40 million folks in america who expertise Sort I allergic reactions. Of this group, during the last three years, roughly 20 million folks have been identified and handled for extreme Sort I allergic reactions which will result in anaphylaxis, however (in 2023, for instance) solely 3.2 million crammed their lively epinephrine auto-injector prescription, and of these, solely half constantly carry their prescribed auto-injector. Even when sufferers or caregivers carry an auto-injector, greater than half both delay or don’t administer the machine when wanted in an emergency.
About ARS Prescription drugs, Inc.
ARS Pharma is a biopharmaceutical firm devoted to empowering at-risk sufferers and their caregivers to raised defend sufferers from allergic reactions that might result in anaphylaxis. The Firm is commercializing neffy® (commerce identify EURneffy® within the EU), an epinephrine nasal spray indicated within the U.S. for emergency remedy of Sort I allergic reactions, together with anaphylaxis, in grownup sufferers and pediatric sufferers 4 years of age and older who weigh 33 lbs. or larger, and within the EU for emergency remedy of allergic reactions (anaphylaxis) because of insect stings or bites, meals, medicinal merchandise, and different allergens in addition to idiopathic or train induced anaphylaxis in adults and kids who weigh 30 kg or larger. For extra data, go to www.ars-pharma.com.
Ahead Trying Statements
Statements on this press launch that aren’t purely historic in nature are “forward-looking statements” inside the that means of the Non-public Securities Litigation Reform Act of 1995. These statements embrace, however aren’t restricted to: expectations and evaluations concerning the medical efficiency and affected person advantages of neffy, together with its needle-free, transportable and straightforward to make use of design; the idea that real-world outcomes knowledge assist the medical interchangeability of neffy and epinephrine injection; and the beliefs that such knowledge will proceed to construct on the medical research of neffy carried out for FDA approval that confirmed neffy achieved blood ranges and pharmacodynamic responses inside the vary of accepted epinephrine injection merchandise and on beforehand revealed medical proof, together with a potential Section 3 examine assessing the efficacy of neffy for the remedy of oral meals challenge-induced anaphylaxis signs; and different statements that aren’t historic reality. As a result of such statements are topic to dangers and uncertainties, precise outcomes might differ materially from these expressed or implied by such forward-looking statements. Phrases reminiscent of “anticipate,” “consider,” “can,” “may,” “anticipate,” “if,” “might,” “potential,” “plan,” “will,” and related expressions are meant to determine forward-looking statements. These forward-looking statements are primarily based upon ARS Pharma’s present expectations and contain assumptions which will by no means materialize or might show to be incorrect.
Precise outcomes and the timing of occasions may differ materially from these anticipated in such forward-looking statements because of varied dangers and uncertainties, which embrace, with out limitation: potential security and different issues from neffy; the power to keep up regulatory approval for neffy in its presently accepted indications; the scope, progress and growth of creating and commercializing neffy; the scope, progress and growth of creating our intranasal epinephrine know-how; medical trial outcomes; the potential for governments and payors to delay, restrict or deny protection for neffy; the scale and development of the marketplace for neffy and the speed and diploma of market acceptance thereof vis-à-vis intramuscular injectable merchandise; ARS Pharma’s capacity to guard its mental property place; and the affect of presidency legal guidelines, laws and insurance policies. Extra dangers and uncertainties that might trigger precise outcomes and outcomes to vary materially from these contemplated by the forward-looking statements are included underneath the caption “Danger Elements” in ARS Pharma’s Quarterly Report on Type 10-Q for the quarter ended March 31, 2025, filed with the Securities and Change Fee (“SEC”) on Might 14, 2025 and in ARS Pharma’s Quarterly Report on Type 10-Q for the quarter ended June 30, 2025, filed with the SEC on August 13, 2025. These paperwork can be accessed on ARS Pharma’s web site at www.ars-pharma.com by clicking on the hyperlink “Financials & Filings” underneath the “Buyers & Media” tab.
The forward-looking statements included on this press launch are made solely as of the date hereof. ARS Pharma assumes no obligation and doesn’t intend to replace these forward-looking statements, besides as required by legislation. For extra data, go to www.ars-pharma.com, and comply with us on LinkedIn and X.
Investor Contact:
Justin Chakma, ARS Pharma
justinc@ars-pharma.com
Media Contact:
Christy Curran, Sam Brown Inc.
christycurran@sambrown.com
615.414.8668
References
1 Patel N, Chong KW, Yip AYG, Ierodiakonou D, Barta J, Boyle RJ, et al. Use of a number of epinephrine doses in anaphylaxis: a scientific overview and meta-analysis. J Allergy Clin Immunol. 2021;148(5):1307-1315.
2 Ebisawa M, Takahashi Ok, Takahashi KK, Yanagida N, Sato S, Lieberman J, et al. Epinephrine nasal spray improves allergic signs in sufferers present process oral meals problem, section 3 trial. J Allergy Clin Immunol. 2025; doi:10.1016/j.jaip.2025.06.038.
Notice of Disclosure: ARS Pharma is an advertiser with SnackSafely.com.